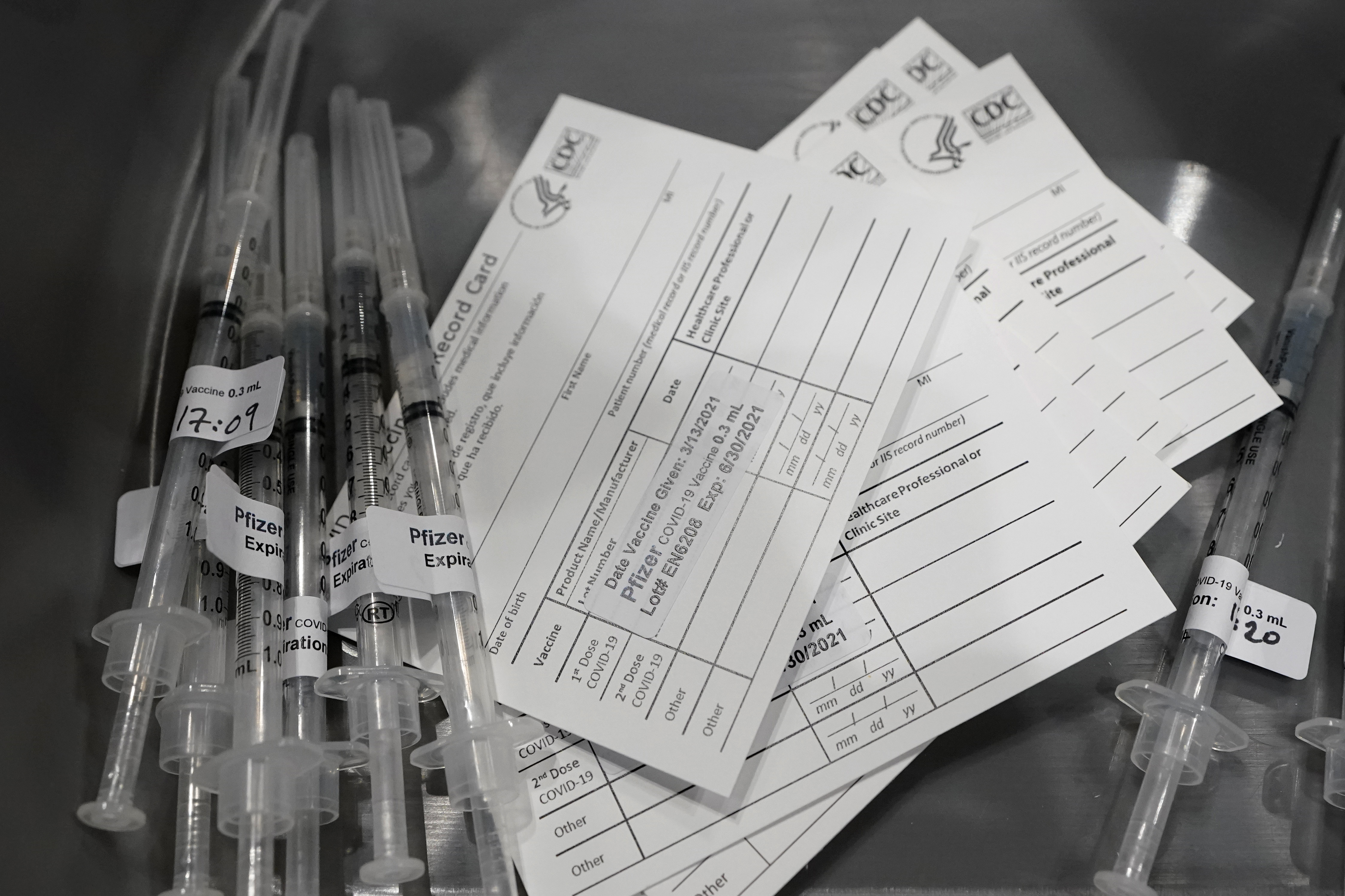
(NEXSTAR) — Drugmaker Pfizer announced Thursday that it has begun testing its COVID-19 vaccine on healthy children ages 6 months to 11 years old.
“Pfizer has deep experience in advancing clinical trials of vaccines in children and infants and is committed to improving the health and well-being of children through thoughtfully designed clinical trials,” the company said in a statement to Nexstar.
Phase 1 begins with an “open-label dose-finding study” to determine the ideal dosage of the vaccine for a total of 144 children.
Once tolerability is confirmed among the participants, the dose will be increased for further study.
The study will begin by testing the vaccine on older children before moving to younger age groups.
The second and third part of the trial will evaluate “the safety, tolerability, and immunogenicity of the selected dose level in each age group,” Pfizer said in a statement. The trial will be randomized, with some participants receiving a placebo.
After a six-month follow-up visit, those who received the placebo will be offered the vaccine.
The Pfizer trial follows COVID-19 vaccine maker Moderna in testing the vaccine on younger children. Moderna announced on March 16 that it had begun testing its vaccine on those under age 12.
It’s not known when the COVID vaccine will be widely available to children.
Dr. Anthony Fauci has previously said that children will likely begin receiving COVID-19 vaccines in the fall.
Speaking to the Wall Street Journal, the nation’s top infectious disease expert said, “We will get children of high school age — 12 to 17 — to get vaccinated by the fall.”
Younger children will likely have to wait longer. Fauci predicted that those under the age of 12 may start receiving the COVID-19 vaccine “in the first part of the first quarter of 2022.”